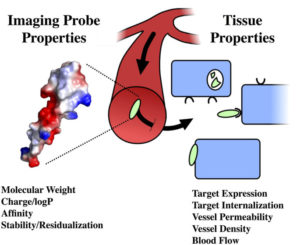
At its core, the Thurber lab is interested in connecting molecular properties to the distribution of drugs and imaging agents across multiple scales – from the whole body down to tissue, single-cell, and subcellular distribution. These molecular properties range from well-known parameters such as charge, molecular weight, and lipophilicity to properties of emerging importance such as colloidal interactions and polyvalent charge impacts on cell signaling. Ultimately, a quantitative and mechanistic computational approach is used to design molecular agents and experimentally test these probes and therapeutics in relevant animal models of disease.
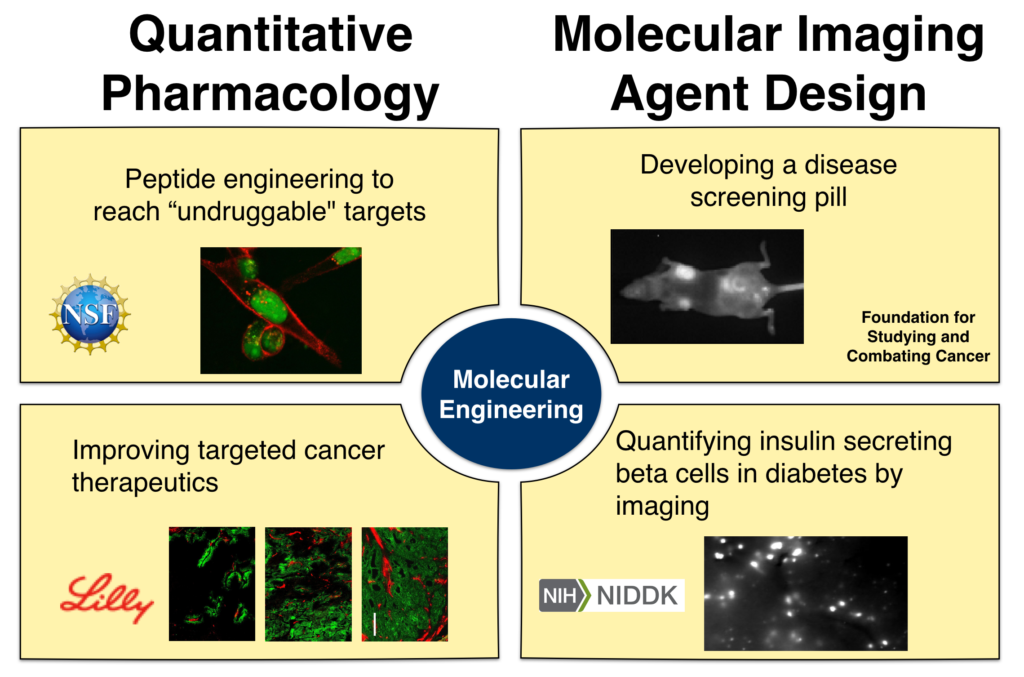
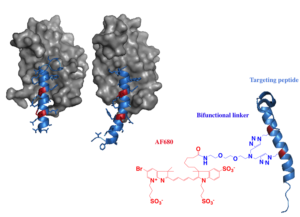
Peptide engineering
Based on estimates from sequencing the human genome, nearly 2/3 of disease associated targets are currently “undruggable” – they are located inside the cell and out of reach for macromolecules but lack a small molecule binding site for inhibition. Stabilized alpha helical drugs lie at the interface of biologics and small molecule drugs, having molecular weight in the 500 to 5,000 Da range. These agents have the potential to reach these targets and disrupt their function without requiring small binding pockets, but the development of these agents is exceedingly complex. The Thurber
Disease screening by self-administered imaging agents
Molecular imaging has transformed our ability to screen for disease and monitor treatment efficacy in a minimally invasive manner with molecular precision. Despite these gains, the technology is limited for some applications from the frequent use of radioactiveprobes and the high cost of advanced imaging techniques. The Thurber lab is exploring self-administration of near-infrared fluorescent probes through subcutaneous injection and/or oral delivery of disease screening agents. These can be administered by the patients and imaged in an office settingfor large scale screening efforts. This paradigm shift from intravenous agents to self-administration should improve patient compliance and lower costs for molecular imaging screens. Computational and experimental approaches are used to ensure adequate contrast to noise ratios at clinical length scales, and predictive probe design is combined with animal imaging to achieve appropriate absorption and clearance for high target to background ratios.
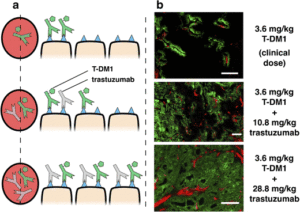
Targeted cancer therapeutics and distribution
Our lab has recently established that tumor distribution of antibody drug conjugates (ADCs), not just potency, plays a major role in efficacy for this drug class. This paradigm shift in development, moving from maximizing potency to a new approach of matching potency with targeting, increases the efficiency of cell killing and has the potential to amplify tumor immunity responses by maximizing antibody coverage of the tumor. Prospective animal studies done in the Thurber lab and an analysis of published literature show that increased penetration consistently improves efficacy in the case of high potency agents.
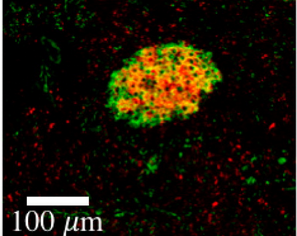
Imaging of pancreatic beta cells in diabetes
Diabetes is a burgeoning disease affecting tens of millions of people in the US. Despite the central role played by the insulin secreting beta cells in the pancreas, there is currently no clinical method for measuring the number of beta cells within a patient. The diabetes community has sought for years to develop an effective imaging agent for quantifying beta cells to study the development of type 1 and type 2 diabetes and monitor treatment response in clinical trials. Unfortunately, the small size of the organ structures where these cells are located (the islets of Langerhans are much smaller than the resolution of clinical imagers) and rarity of the cells (forming only ~1% of the pancreas) make imaging a challenging task. Using detailed measurements of the pancreas physiology and receptor trafficking, the Thurber lab is designing imaging approaches to improve the specificity and sensitivity of some of the most promising agents to enable accurate beta cell quantification in the clinic.